- Visibility 1k Views
- Downloads 55 Downloads
- Permissions
- DOI 10.18231/j.jdp.2024.027
-
CrossMark
- Citation
Analyzing guided bone regeneration methods: A review of the literature
Abstract
Guided bone regeneration (GBR) is a surgical technique involving bone grafts and barrier membranes to repair minor defects near dental implants. It is typically used for dehiscence or fenestration defects ≥2 mm, often incorporating autogenous bone for larger defects. Achieving tension-free primary closure is essential to avoid wound dehiscence, a leading cause of GBR failure. Proper fixation of the barrier membrane without mobility is crucial. Continuous monitoring is necessary if the membrane becomes exposed to prevent secondary infections.
Guided Bone Regeneration
Guided Bone Regeneration (GBR) is a bone graft procedure involving a barrier membrane to prevent soft tissue invasion during the repair bone of defects around dental implants. Bone tissue possesses significant regenerative potential, yet various factors such as vascular supply failure, mechanical instability, oversized defects, and high-proliferative competing tissues can hinder this process.[1]
Techniques to enhance bone formation include growth factor-induced osteoinduction, osteoconduction via autogenous bone grafts or substitutes, stem cell transfer for osteoblast differentiation, distraction osteogenesis, and GBR using barrier membranes in conjunction with bone grafting materials. GBR, often combined with bone grafts, is widely utilized in routine dental practice[2] to augment bone. The decision to employ GBR depends on the extent of remaining bone walls post-extraction; it becomes crucial in cases of significant bone loss [2] or large defects.
Principle of GBR
The core concept of GBR is to prevent unwanted cells from non-bone tissues from impeding bone regeneration. A physical barrier membrane is placed between the area targeted for new bone growth and nearby soft tissues. Bone grows relatively slowly compared to fibroblasts and epithelial cells, which can quickly occupy space during wound healing and form connective tissue faster than bone can regenerate.
If the barrier membrane remains intact and is not exposed to the oral cavity for a sufficient period, ideal conditions are created for blood vessels from existing bone to penetrate into the area. This allows stem cells and osteoprogenitor cells to differentiate into osteoblasts, facilitating the production of bone matrix. Essentially, the barrier membrane creates a protected space that harnesses the bone's inherent healing capabilities undisturbed.
Key Factors for Achieving Stable Long-Term Results
Achieving predictable long-term outcomes in guided bone regeneration (GBR) procedures hinges on several critical factors identified by Buser and Chen, originally outlined for implant placement in post-extraction sites, and relevant to GBR as well.
Implant Surgeon: The foremost factor is the skill and decision-making ability of the implant surgeon. A thorough patient assessment, appropriate biomaterial selection, and choosing the optimal surgical approach are crucial for achieving anticipated treatment outcomes. The surgeon's education, surgical proficiency, and experience with GBR procedures are vital.[3]
Patient Risk Assessment: A comprehensive evaluation of the patient's health profile determines the risk level associated with the procedure. Factors such as smoking history, previous periodontitis, and oral hygiene practices significantly impact long-term peri-implant tissue stability. Regular supportive maintenance care every 3 to 6 months is recommended, especially for patients with high-risk profiles due to systemic conditions like diabetes mellitus or rheumatic disorders.[3], [4]
Local Bone Anatomy: The morphology of the defect and local bone anatomy influence the choice between simultaneous or staged GBR procedures. Advances in 3D radiographic imaging, such as CBCT scans, aid in precise preoperative planning.[5]
Selection of Surgical Approach: Based on preoperative assessment and patient consent, the surgical approach—whether simultaneous or staged, and horizontal or vertical bone augmentation—is determined. [5]
Choice of Biomaterials: Selecting appropriate biomaterials, including dental implants, bone grafts, substitutes, and barrier membranes, is critical for the success and long-term stability of GBR procedures. [5], [6]
Implant Selection: The type, material, surface characteristics, shape, diameter, and length of the implant play pivotal roles in achieving long-term stability and success. Evidence-based selection criteria for implants should be applied to ensure optimal outcomes. [7]
By addressing these key factors systematically, clinicians can enhance the predictability and longevity of outcomes in guided bone regeneration procedures, ensuring successful implant therapy and patient satisfaction over the long term.
In addition to anatomical considerations, essential surgical factors significantly influence the success of GBR procedures. These factors include:
Selection of Bone Grafts and Substitutes: Historically, autogenous bone chips were initially used to prevent membrane collapse mechanically under barrier membranes. Subsequently, various bone grafts and substitutes have been studied to understand their osteogenic potential and substitution rates crucial for GBR.[8], [9]
Osteogenic Potential: Bone fillers with high osteogenic potential accelerate new bone formation early in the healing process by releasing growth factors like TGF-β1 and BMP-2 into the surrounding environment.[9]
Substitution Rate: The rate at which a bone filler is resorbed and replaced by new bone during remodeling impacts long-term stability. Fillers with high substitution rates initially favored for rapid integration, were later reconsidered due to clinical observations of volume reduction over time. This led to a shift towards fillers with lower substitution rates, such as hydroxyapatite-derived materials.[9], [10]
Composite Grafts: Combining fillers with complementary characteristics—such as highly osteogenic autogenous bone chips and low substitution rate materials like deproteinized bovine bone mineral (DBBM)—as composite grafts has become standard practice. This approach aims to optimize bone augmentation and ensure stable, long-term results.[11], [12]
Bone-Conditioned Medium (BCM): Autogenous bone chips can be stored in a mixture of blood and Ringer’s solution to create BCM, which bioactivates bone substitutes and barrier membranes, enhancing their performance in promoting bone regeneration [12], [13].
Leading GBR surgeons globally now commonly utilize composite grafts due to their synergistic benefits, adapting their approach based on defect anatomy and individual preferences. These advancements underscore the ongoing evolution in optimizing surgical strategies for effective bone regeneration and long-term implant success.
Biomaterials Used in Guided Bone Regeneration (GBR)
Barrier membrane selection
Initially, expanded polytetrafluoroethylene (ePTFE), branded as GORE-TEX by Gore Medical, was standard for GBR until the mid-1990s. ePTFE, a non-resorbable and bioinert membrane, required a second surgery for removal and posed challenges like hydrophobicity, necessitating fixation and sometimes causing soft tissue complications during healing.
In response, efforts focused on finding resorbable alternatives, intensively discussed at a 1993 meeting in Arizona.[14] Options included polymeric membranes (polylactic or polyglycolic acid) and collagen membranes from animal sources. By the late 1990s, collagen-based membranes proved most effective in studies.
Today, collagen dominates the GBR market, while bioinert PTFE membranes remain for complex cases like vertical ridge augmentation. The original ePTFE is replaced by various PTFE alternatives. Membrane selection now considers defect morphology, procedure goals (horizontal vs vertical augmentation), and surgeon preference. [15]
Barrier membranes
Barrier membranes are crucial in guided bone regeneration (GBR) procedures, placed directly over bone defects to prevent soft tissue ingrowth and facilitate bone regeneration. Clinicians choose from a variety of materials based on specific clinical needs, considering key properties such as biocompatibility, cell occlusion, space maintenance, tissue integration, degradability, clinical handling, and susceptibility to complications. [16]
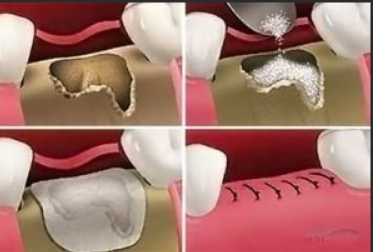
Non-resorbable membrane
Initially, non-resorbable membranes like expanded polytetrafluoroethylene (e-PTFE) were standard, known for their mechanical barrier properties. However, they required a second surgery for removal, posed handling challenges due to hydrophobicity, and risked soft tissue dehiscence during healing, affecting regenerative outcomes.[17], [18] High-density PTFE (d-PTFE), developed to address these issues with smaller pore sizes, showed improved clinical outcomes and easier removal. [19], [20]
Titanium mesh
For maintaining space in severe osseous defects, titanium mesh has become a reliable option. Its flexibility and ability to resist soft tissue pressure make it suitable for ridge augmentation and maintaining bone volume around implants. It allows for proper blood supply and can be used either before or during implant placement to enhance bone regeneration. [21], [22]
Titanium-reinforced PTFE
Titanium-reinforced e-PTFE or d-PTFE membranes offer additional stability and shaping capabilities for large defects. Studies demonstrate their effectiveness in maintaining space, promoting bone formation, and preserving ridge morphology during healing, making them suitable for complex cases. [23], [24]
Resorbable membrane
Bioresorbable membranes, including synthetic polymers like polyglycolides (PGA) and polylactides (PLA), as well as collagen membranes derived from animal sources, provide advantages such as eliminating the need for removal surgery, reducing patient morbidity, and enhancing tissue integration. However, they require careful consideration of degradation rates to ensure proper barrier function and support for bone regeneration. [25], [26]
Collagen membranes
Collagen membranes, derived from various animal tissues, are popular for their biocompatibility, easy handling, and ability to support bone formation. Cross-linking can modify their properties, affecting degradation rates and tissue response. Rapid degradation in the oral environment can lead to spontaneous healing but may compromise barrier function if too fast. [27], [28]
Comparison and considerations
Choosing between resorbable and non-resorbable membranes depends on clinical requirements like defect size, bone augmentation goals, and patient-specific factors. Non-resorbable membranes excel in space maintenance and bone formation predictability but require second surgeries and may pose infection risks. Resorbable membranes offer convenience and reduced morbidity but need careful management of degradation rates and may lack rigidity for vertical bone augmentation. [29], [30]
Future directions
Advancements in tissue engineering aim to develop functional membranes that induce direct bone regeneration, potentially combining the advantages of both resorbable and non-resorbable materials to optimize clinical outcomes in implant dentistry. [31]
Grafting materials overview
Bone regeneration utilizes osteogenesis (bone formation), osteoinduction (transforming stem cells into bone cells), and osteoconduction (providing a scaffold for new bone formation). Autogenous bone, allografts (from cadavers), xenografts (animal-derived), and alloplasts (synthetic) each leverage these mechanisms. [32], [33]
Autogenous bone is osteogenic, osteoinductive, and osteoconductive, harvested from the patient. Allografts lack osteogenic potential but offer osteoconductive properties. Xenografts and alloplasts are osteoconductive but not osteogenic. Combining graft types optimizes bone regeneration outcomes
Techniques like autogenous bone chip harvesting enhance grafting success. Allografts, xenografts, and alloplasts serve as alternatives when autogenous bone is insufficient. Membrane stabilization and graft stability are critical for effective guided bone regeneration (GBR).
Biomaterial-based delivery
Biomaterials play a crucial role in delivering growth factors (GFs) and stem cells for tissue regeneration. Growth factors like VEGFs, FGFs, PDGFs, and IGFs control cell functions such as proliferation and differentiation. PDGF and FGF are particularly significant in bone regeneration and angiogenesis. Biomaterials like sponges, nanoparticles, and hydrogels are used to physically or chemically bind and release GFs, enhancing their therapeutic efficacy in bone growth and repair. [34]
Stem cell delivery
Biomaterials facilitate the delivery of stem cells from sources like adipose tissue and bone marrow, aiding in tissue regeneration without immune rejection. Hydrogels are particularly effective in minimally invasive delivery for craniofacial deformities and disorders.
Gene selivery
Genes encoding growth factors are delivered via biomaterials to stimulate tissue repair, overcoming limitations of short GF half-lives. Studies show successful gene transfer in periodontal regeneration using biomaterials, although further research is needed on safety and efficacy.
Scaffold and cell-free technologies
Extracellular vesicles (EVs), including exosomes, are explored for their therapeutic potential in tissue regeneration, leveraging MSC-derived components in various diseases. Two-dimensional materials like MoS2 and BP show promise in biomedical applications for their unique properties, enhancing cell-material interactions and potential in medical devices. Overall, biomaterials are pivotal in advancing therapies across orthopedics, periodontics, and dentistry, offering tailored solutions for tissue regeneration and disease treatment.
GBR Uses
GBR for maxillary implants
In treating the anterior maxilla, long-term stability of peri-implant tissues and achieving pleasing esthetic outcomes are primary goals. Following these are ensuring proper function and speech. Our preferred method involves early implant placement (Type 2) after soft tissue healing, which has proven highly successful over 20 years. [35]
This approach is chosen for extraction sites with thin facial bone walls and allows for precise 3D implant positioning with good primary stability. It's particularly suited for cases where the resulting bone defect shows a two-wall morphology on the facial aspect, common in anterior maxillary extractions. Type 2 placement constitutes over 80% of our procedures.
Post soft tissue healing, an open-flap procedure is performed for contour augmentation using GBR, significantly reducing the need for additional soft tissue grafting. This method utilizes a two-layer composite graft with autogenous bone chips for accelerated bone formation and DBBM particles for long-term volume stability.
To avoid a second flap procedure for membrane removal, we prefer resorbable barriers like non-cross-linked collagen membranes in most cases. This approach not only offers practical advantages but also reduces patient discomfort and costs.Fixation pins and tacks are occasionally used in vertical augmentation or the sausage technique but are unnecessary for routine GBR in the maxilla.
Guided bone regeneration (GBR) success depends on several factors
Patient conditions like smoking, flap design with mid-crestal and mesial incisions for optimal healing due to the edentulous ridge's avascular zone, tension-free flap advancement via periosteal releasing incisions (PRI), and stable membrane fixation using techniques such as screws or cover screws. [36]
Decortication, though beneficial for graft integration, may slightly extend surgery time and increase postoperative discomfort. Attention to these factors is crucial for enhancing GBR outcomes.
Guided bone regeneration (GBR) with simultaneous implant placement is recommended only when the implant can achieve optimal three-dimensional positioning and satisfactory primary stability in existing natural bone. [37]
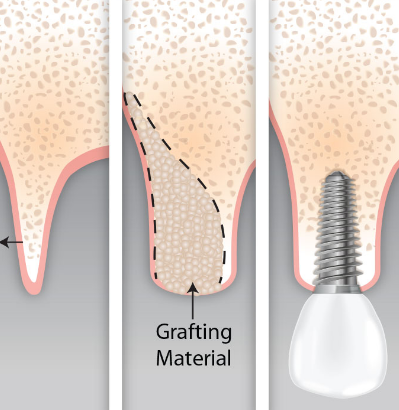
GBR is often performed as a staged approach when ridge anatomy prevents ideal three-dimensional implant placement initially. This two-step procedure involves first performing hard tissue reconstruction before subsequent implant placement.
Studies suggest that GBR using membranes and bone substitutes can successfully regenerate bone, with implant placement typically planned five to nine months post-GBR. This approach yields predictable results, particularly in Cawood classes III and IV.
For vertical bone augmentation before implants, Jovanovic et al. (1995) recommend non-resorbable e-PTFE membranes with DFDBA or DBBM, possibly combined with autogenous chips.
Key challenges in this procedure include membrane exposure and soft tissue collapse, necessitating tension-free flap closure and precise suturing techniques. Tenting screws have proven effective in minimizing soft tissue collapse. [38]
GBR Success Criteria
The success of guided bone regeneration (GBR) is crucial in implant dentistry. Traditionally, success was defined as covering a dehisced or fenestrated implant surface with regenerated hard tissue.


Second-generation definition emphasized regenerating bone of sufficient dimension to withstand functional forces long-term [39]
However, these definitions are now considered inadequate. In the aesthetic zone, success must ensure regeneration of pre-pathological alveolar ridge morphology. This supports soft tissue coverage and maximizes treatment outcomes, marking the third-generation definition of GBR success.
Source of Funding
None.
Conflict of Interest
None.
References
- Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9(4):290-6. [Google Scholar]
- Nyman S, Gottlow J, Karring T, Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. 1982;9(3):257-65. [Google Scholar]
- Ray J, Doddi N, Regula D, Williams J, Melveger A. Polydioxanone (PDS), a novel monofilament synthetic absorbable suture. Surg Gynecol Obstet. 1981;153(4):497-507. [Google Scholar]
- Hürzeler M, Quiñones C, Schüpbach P. Guided bone regeneration around dental implants in the atrophic alveolar ridge using a bioresorbable barrier. An experimental study in the monkey. Clin Oral Implants Res. 1997;8(4):323-31. [Google Scholar]
- Hutmacher D, Hürzeler M, Schliephake H. A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int J Oral Maxillofac Implants. 1996;11(5):667-78. [Google Scholar]
- Vert M, Li S, Garreau H. New insights on the degradation of bioresorbable polymeric devices based on lactic and glycolic acids. Clin Mater. 1992;10(1-2):3-8. [Google Scholar]
- Vert M, Mauduit J, Li S. Biodegradation of PLA/GA polymers: Increasing complexity. Biomaterials. 1994;15(5):1209-13. [Google Scholar]
- Misch C, Dietsh F. Bone-grafting materials in implant dentistry. Implant Dent. 1993;2(3):158-67. [Google Scholar]
- Evian C, Rosenberg E, Coslet J. The osteogenic activity of bone removed from healing extraction sockets in humans. J Periodontol. 1982;53:81-5. [Google Scholar] [Crossref]
- Rose L, Mb, Genco R, Cohen W. . Periodontics: Medicine, Surgery, and Implants. 2004. [Google Scholar]
- Barboza E, Caula A, Machado F. Potential of recombinant human bone morphogenetic protein-2 in bone regeneration. Implant Dent. 1999;8(4):360-7. [Google Scholar]
- Wang E, Rosen V, D'Alessandro J, Bauduy M, Cordes P, Harada T. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87(6):2220-4. [Google Scholar]
- Toriumi D, Kotler H, Luxenberg D. Mandibular reconstruction with a recombinant bone-inducing factor. Functional, histologic, and biomechanical evaluation. Arch Otolaryngol Head Neck Surg. 1991;117(10):1101-12. [Google Scholar]
- Garg A. Bone Biology, Harvesting and Grafting for Dental Implants: Rationale and Clinical Applications. . 2004. [Google Scholar]
- Zaner D, Yukna R. Particle size of periodontal bone grafting materials. J Periodontol. 1984;55(7):406-9. [Google Scholar]
- Burchardt H. Biology of bone transplantation. Orthop Clin North Am. 1987;18(2):187-96. [Google Scholar]
- Tatum O. Osseous grafts in intra-oral sites. J Oral Implant. 1996;22(1):51-2. [Google Scholar]
- Schallhorn R. Postoperative problems associated with iliac transplants. J Periodontol. 1972;43(1):3-9. [Google Scholar]
- Dragoo M, Sullivan H. A clinical and histological evaluation of autogenous iliac bone grafts in humans. II. External root resorption. J Periodontol. 1973;44(10):614-25. [Google Scholar]
- Quattlebaum J, Mellonig J, Hensel N. Antigenicity of freezedried cortical bone allograft in human periodontal osseous defects. J Periodontol. 1988;59(6):394-7. [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-9. [Google Scholar]
- Mellonig J, Bowers G, Bailey R. Comparison of bone graft materials. Part I. New bone formation with autografts and allografts determined by Strontium-85. J Periodontol. 1981;52(6):291-6. [Google Scholar]
- Mellonig J, Bowers G, Cotton W. Comparison of bone graft materials. Part II. New bone formation with autografts and allografts: a histological evaluation. J Periodontol. 1981;52(6):297-302. [Google Scholar]
- Schwartz Z, Mellonig J, Carnes D, Fontaine J, Cochran D, Dean D. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1996;67(9):918-26. [Google Scholar]
- . Committee on Research, Science and Therapy of the American Academy of Peridontology. Tissue banking of bone allografts used in periodontal regeneration. J Periodontol. 2001;72(6):834-8. [Google Scholar]
- Sanders J, Sepe W, Bowers G, Koch R, Williams J, Lekas J. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. Part III. Composite freeze-dried bone allografts with and without autogenous bone grafts. J Periodontol. 1983;54(1):1-8. [Google Scholar]
- Steenberghe DV, Callens A, Geers L, Jacobs R. The clinical use of deproteinized bovine bone mineral on bone regeneration in conjunction with immediate implant installation. Clin Oral Implants Res. 2000;11(3):210-6. [Google Scholar]
- Piattelli M, Favero G, Scarano A, Orsini G, Piattelli A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: a histologic long-term report of 20 cases in humans. Int J Oral Maxillofac Implants. 1999;14(6):835-40. [Google Scholar]
- Bunyaratavej P, Wang H. Collagen membranes: A review. J Periodontol. 2001;72(2):215-29. [Google Scholar]
- Hammerle C, Jung R. Bone augmentation by means of barrier membranes. Periodontology. 2000;33:36-53. [Google Scholar] [Crossref]
- Dahlin C, Gottlow J, Linde A. Healing of maxillary and mandibular bone defects using a membrane technique. An experimental study in monkeys. Scand J Plast Reconstr Surg Hand Surg. 1990;24(1):13-9. [Google Scholar]
- Buser D, Dula K, Belser U, Hirt H, Berthold H. Localized ridge augmentation using guided bone regeneration. 1. Surgical procedure in the maxilla. Int J Periodontics Restorative Dent. 1993;13(1):29-45. [Google Scholar]
- Becker W, Lynch S, Lekholm U, Becker B, Caffesse R, Donath K. A comparison of ePTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freezedried bone in promoting bone formation around immediate extraction socket implants. J Periodontol. 1992;63(11):929-40. [Google Scholar]
- Walters S, Greenwell H, Hill M, Drisko C, Pickman K, Scheetz J. Comparison of porous and non-porous teflon membranes plus a xenograft in the treatment of vertical osseous defects: a clinical reentry study. J Periodontol. 2003;74(8):1161-8. [Google Scholar]
- Buser D, Dula K, Hirt HP. Lateral ridge augmentation using autografts and barrier membranes: a clinical study with 40 partially edentulous patients. J Am Assoc Oral Maxillofac Sur. 1996;54(4):420-32. [Google Scholar]
- Simon B, Hagen S, Deasy M, Faldu M, Resnansky D. Changes in alveolar bone height and width following ridge augmentation using bone graft and membranes. J Periodontol. 2000;71(11):1774-91. [Google Scholar]
- Simion M, Maglione M, Iamoni F, Scarano A, Piattelli A, Salvato A. Bacterial penetration through Resolut resorbable membrane in vitro: an histological and scanning electron microscopic study. Clin Oral Implants Res. 1997;8(1):23-31. [Google Scholar]
- Wang H, Carroll M. Guided bone regeneration using bone grafts and collagen membranes. Quint Int. 2001;32(7):504-15. [Google Scholar]
- Postlethwaite A, Seyer J, Kang A. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagenderived peptides. Proc Natl Acad Sci U S A. 1978;75(2):871-5. [Google Scholar]
- Abstract
- Guided Bone Regeneration
- Key Factors for Achieving Stable Long-Term Results
- Biomaterials Used in Guided Bone Regeneration (GBR)
- Barrier membrane selection
- Barrier membranes
- Non-resorbable membrane
- Titanium mesh
- Titanium-reinforced PTFE
- Resorbable membrane
- Collagen membranes
- Comparison and considerations
- Future directions
- Grafting materials overview
- Biomaterial-based delivery
- Stem cell delivery
- Gene selivery
- Scaffold and cell-free technologies
- GBR Uses
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Bharat A, B AA, Sharma AK, Sharma V, Bharat P, Bharat A. Analyzing guided bone regeneration methods: A review of the literature [Internet]. J Dent Panacea. 2024 [cited 2025 Oct 30];6(3):130-135. Available from: https://doi.org/10.18231/j.jdp.2024.027
APA
Bharat, A., B, A. A., Sharma, A. K., Sharma, V., Bharat, P., Bharat, A. (2024). Analyzing guided bone regeneration methods: A review of the literature. J Dent Panacea, 6(3), 130-135. https://doi.org/10.18231/j.jdp.2024.027
MLA
Bharat, Anjali, B, Aravind Anto, Sharma, Amit Kumar, Sharma, Vikram, Bharat, Pooja, Bharat, Archana. "Analyzing guided bone regeneration methods: A review of the literature." J Dent Panacea, vol. 6, no. 3, 2024, pp. 130-135. https://doi.org/10.18231/j.jdp.2024.027
Chicago
Bharat, A., B, A. A., Sharma, A. K., Sharma, V., Bharat, P., Bharat, A.. "Analyzing guided bone regeneration methods: A review of the literature." J Dent Panacea 6, no. 3 (2024): 130-135. https://doi.org/10.18231/j.jdp.2024.027
