- Visibility 1.4k Views
- Downloads 39 Downloads
- Permissions
- DOI 10.18231/j.jdp.2024.039
-
CrossMark
- Citation
Comparison between QR678 neo and platelet rich plasma for hair growth -A retrospective study
Abstract
Background: Androgenetic alopecia is the most common cause of hair loss, affecting self-esteem and quality of life. While platelet-rich plasma has been a standard treatment, it yields inconsistent results due to patient variability. QR678 Neo, a biomimetic peptide-based treatment, offers a standardized alternative.
Aims and Objectives: This study aimed to compare the efficacy and safety profiles of QR678 Neo and platelet-rich plasma in treating androgenetic alopecia. Objectives included evaluating improvements in hair density, hair quality, patient satisfaction, and reduction in hair fall.
Materials and Methods: This retrospective study compared the efficacy of QR678 Neo and platelet-rich plasma in 70 AGA patients. The study evaluated hair density, quality, and patient satisfaction over 6 months.
Results: QR678 Neo showed a 30% improvement in hair density versus 18% with platelet-rich plasma (p<0.01). Patients treated with QR678 Neo reported superior satisfaction (VAS score 9.2 vs. 7.8).
Conclusion: QR678 Neo outperformed PRP in all evaluated parameters, offering a consistent and effective alternative for Androgenetic alopecia treatment.
Introduction
Androgenetic alopecia (AGA), commonly referred to as male or female pattern baldness, is the most prevalent cause of hair loss worldwide. It affects nearly 50% of men and women by the age of 50, with a progressive onset that begins as early as the teenage years in some individuals. AGA is characterized by the miniaturization of hair follicles due to the effects of dihydrotestosterone (DHT) on genetically susceptible follicles, leading to a gradual thinning of hair and eventual baldness in affected regions. This condition not only alters physical appearance but also significantly impacts psychological well-being, self-esteem, and overall quality of life, making it a major concern for affected individuals. [1]
Current treatment modalities for AGA aim to slow progression, stimulate hair regrowth, or both. Widely used therapies include topical minoxidil and oral finasteride. While these options have demonstrated efficacy, they often come with limitations such as side effects, inconsistent results, and the need for long-term adherence to maintain their effects. Platelet-rich plasma (PRP) therapy has also gained popularity as a minimally invasive option. PRP utilizes autologous platelets to release growth factors that stimulate hair follicle activity and improve vascularization. However, PRP outcomes are highly dependent on patient variability and platelet quality, leading to inconsistent treatment responses. These challenges underscore the need for alternative, more reliable, and standardized therapies. [1], [2], [3]
QR678 Neo – short for "Quick Response to a disease which earlier had no answer" – represents a novel approach in the treatment of hair loss. It is an advanced hair growth formulation comprising biomimetic peptides that mimic the functions of naturally occurring growth factors involved in hair follicle stimulation and angiogenesis. Key components include vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and insulin-like growth factor-1 (IGF-1), all of which play critical roles in hair follicle cycling and vascular support. Unlike PRP, QR678 Neo ensures consistent and standardized efficacy, as it is not dependent on individual patient factors such as platelet quality. This attribute addresses a significant limitation of PRP and other autologous treatments. [4], [5], [6]
Recent studies have highlighted QR678 Neo's effectiveness in various forms of alopecia. Kapoor et al.[7] demonstrated its potential in treating chemotherapy-induced hair loss, while Shome et al.[8] reported its success in addressing female pattern hair loss. These findings underscore QR678 Neo’s versatility and effectiveness across diverse patient populations. However, while QR678 Neo has shown promising results, direct comparisons with established treatments like PRP remain limited in scope.
This study aims to bridge this gap by conducting a direct comparison of QR678 Neo and PRP in patients with AGA. Specifically, the study seeks to evaluate the efficacy and safety of QR678 Neo relative to PRP in promoting hair regrowth, improving hair density, and enhancing patient satisfaction. By exploring the advantages and limitations of both treatments, this research aims to provide robust evidence to guide clinical decision-making and optimize outcomes for individuals affected by androgenetic alopecia.
Materials and Methods
Study design
This retrospective study included 70 patients diagnosed with androgenetic alopecia (grades II-IV based on the Norwood scale) who sought treatment at La Densitae Hair, Skin, and Laser Clinic between July 2023 and July 2024. The patients were divided into two groups based on the treatment modality received: the QR678 Neo group and the PRP group. Patients in the QR678 Neo group underwent four to six sessions of intradermal injections of QR678 Neo administered at 1 month intervals. Meanwhile, patients in the PRP group received four to six sessions of intradermal injections of platelet-rich plasma (PRP), prepared using standard double-spin centrifugation protocols.
Participants were selected based on specific inclusion and exclusion criteria. The inclusion criteria were an age range of 20-45 years, a diagnosis of androgenetic alopecia (grades II-IV), and no prior hair loss treatment in the preceding six months. Exclusion criteria included the presence of autoimmune or systemic diseases, scalp infections or keloidal tendencies, and a history of allergic reactions to growth factors.
The primary outcome measures were hair density, evaluated via trichoscopy, and patient satisfaction, assessed using a visual analog scale (VAS). Secondary outcomes included hair quality, assessed through patient-reported evaluations, and a reduction in hair fall, measured by the hair pull test.
Statistical analysis
Data were analyzed using SPSS v25. A paired t-test evaluated intragroup differences, while independent t-tests assessed intergroup comparisons. A p-value <0.05 was considered statistically significant.
Results
Demographics
The participants had a mean age of 32.6 years, with an equal distribution of male and female participants. Both groups were well-matched in terms of baseline characteristics, including the severity of androgenetic alopecia based on the Norwood-Hamilton scale.
Primary outcomes
Hair density
QR678 Neo demonstrated a 30% mean increase in hair density, improving from a baseline of 120 follicles/cm² to 156 follicles/cm² post-treatment.
The PRP group showed an 18% mean increase, with hair density rising from 122 follicles/cm² to 144 follicles/cm² post-treatment.
The differences between the two groups were statistically significant (p < 0.01).
Patient satisfaction
Participants in the QR678 Neo group rated their satisfaction at a mean VAS score of 9.2/10.
he PRP group reported a lower mean VAS score of 7.8/10.
This difference in patient satisfaction was statistically significant (p < 0.05).
Secondary outcomes
Hair quality
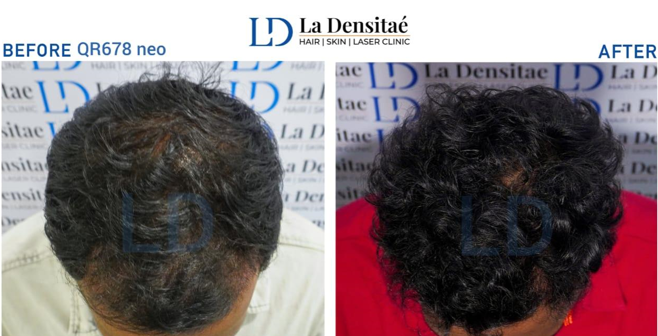
Patients treated with QR678 Neo reported thicker and smoother hair strands, with consistent improvements noted across the group. ([Figure 1])
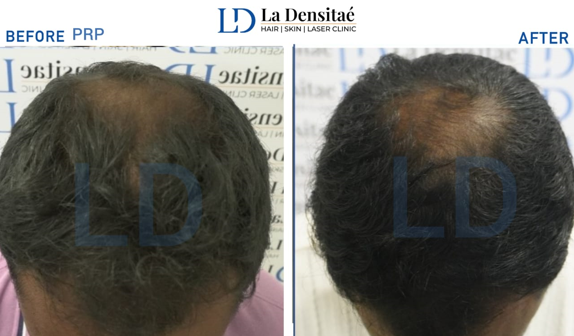
The PRP group experienced moderate improvements in hair quality, but with less uniform results.([Figure 2])
Reduction in Hair Fall
Hair pull tests were negative in 90% of patients treated with QR678 Neo, indicating a substantial reduction in hair fall.
In comparison, only 60% of PRP-treated patients had negative hair pull tests, suggesting a less effective reduction in hair fall.
These outcomes collectively highlight QR678 Neo's superior efficacy compared to PRP in treating androgenetic alopecia. ([Figure 3])
![Comparison of hair density increases between QR678 Neo and PRP treatments and VAS scores and hair fall reduction percentages for QR678 Neo and PRP groups.]](https://s3-us-west-2.amazonaws.com/typeset-prod-media-server/ade41e06-d63a-47e9-a8f3-6c23d055ac68image3.png)
Discussion
QR678 Neo’s formulation capitalizes on biomimetic peptides designed to mimic the actions of naturally occurring growth factors. VEGF enhances blood vessel formation around hair follicles, ensuring optimal nutrient supply. IGF-1 promotes cellular proliferation, delaying follicular apoptosis, while bFGF initiates and sustains the anagen phase. These mechanisms are supported by earlier research on the role of growth factors in hair follicle biology. [4], [3], [5], [9]
The findings of this study highlight the superiority of QR678 Neo over PRP in promoting hair regrowth and improving patient satisfaction. The 30% improvement in hair density observed with QR678 Neo significantly outperformed the 18% improvement achieved with PRP (p < 0.01). These results align with earlier studies such as those by Kapoor and Shome, which demonstrated QR678 Neo’s consistent efficacy in various forms of alopecia, including female pattern hair loss and chemotherapy-induced alopecia. [1], [4], [10] Unlike PRP, which relies on patient-derived platelets, QR678 Neo’s biomimetic peptides ensure a standardized response, addressing variability issues often associated with PRP treatments. [2], [11]
Patient satisfaction, measured through a visual analog scale, was markedly higher in the QR678 Neo group (mean score 9.2/10) compared to the PRP group (mean score 7.8/10). This corroborates findings from Shome et al., who reported superior patient-reported outcomes with QR678 Neo in randomized trials. [3] The higher satisfaction scores can be attributed to QR678 Neo’s ability to promote uniform hair regrowth and improve hair strand quality. In contrast, PRP treatments, as noted by Bhargava et al., often yield less predictable outcomes due to variations in platelet concentration and growth factor activation. [2] Additionally, studies by Li et al. further validate the predictable outcomes of biomimetic peptides compared to PRP. [7], [12]
In terms of safety, QR678 Neo demonstrated an excellent profile, with no adverse effects reported during the trial. Cytotoxicity studies conducted by Shome et al. further confirmed the formulation’s biocompatibility, making it a safer alternatIve to PRP, which occasionally causes scalp irritation or inflammation due to the variability in platelet quality. [3], [7] This consistent safety profile positions QR678 Neo as a viable treatment option for patients with autoimmune disorders or sensitivity to conventional therapies. Studies such as those by Kapoor et al. have emphasized the importance of safety in therapeutic innovations for alopecia. [10], [13]
These findings are consistent with the broader literature, which has increasingly emphasized the limitations of PRP in terms of patient variability and inconsistent outcomes. Studies by Yano et al. and Philpott et al. have underscored the importance of growth factors like VEGF and IGF-1 in promoting angiogenesis and follicular stimulation, mechanisms that QR678 Neo directly targets through its peptide formulation. [6], [8], [9] By contrast, PRP treatments are contingent on individual platelet quality, as highlighted by Kapoor et al., leading to variable results that complicate their clinical application. [1], [2], [11]
While this study provides robust evidence supporting QR678 Neo’s efficacy, it is not without limitations. The retrospective nature of the study and the sample size of 70 participants, while larger than many prior studies, may still limit generalizability. Additionally, the follow-up period was relatively short, and cost considerations were not addressed. Larger-scale studies with extended observation periods are essential to validate these results and assess long-term efficacy. Exploring the cost-effectiveness of QR678 Neo relative to other emerging therapies such as stem cell treatments or laser-assisted hair regrowth could further substantiate its clinical utility. Bhargava et al. have previously noted the need for comparative studies to assess the economic feasibility of such innovative therapies in routine clinical practice. [2], [14], [15]
Future research should also explore the broader application of QR678 Neo for other types of alopecia and in more diverse patient populations, encompassing variations in age, ethnicity, and hair type. Additionally, emerging technologies like Biotin-enriched PRP, GFC, stem cell treatments, and laser-based modalities could provide deeper insights into effective combination therapies for hair restoration. Exploring the long-term sustainability of results, particularly in comparison with advanced techniques like exosome therapy, may also add depth to the existing literature.
In conclusion, the biomimetic approach of QR678 Neo circumvents the variability and limitations inherent to PRP, providing a consistent and predictable therapeutic option. These findings, together with supporting evidence from prior studies, suggest that QR678 Neo could redefine the standards for hair restoration therapy.
Conclusion
QR678 Neo represents a significant advancement in hair restoration, out per forming PRP in promoting hair density, quality, and patient satisfaction. Its biomimetic peptide formulation ensures consistent efficacy and safety, establishing it as a superior therapeutic option for androgenetic alopecia. However, further research is warranted to explore its broader applications and confirm its long-term benefits in diverse patient populations.
Ethical Considerations
Ethical committee clearance was not obtained for this study as it is a retrospective analysis of data collected from July 2023 to July 2024. All patient data were anonymized, and the study adhered to the principles outlined in the Declaration of Helsinki.
Source of Funding
None.
Conflict of Interest
None declared.
References
- Kapoor R, Shome D. Intradermal injections of a hair growth factor formulation for enhancement of human hair regrowth: Safety and efficacy evaluation in a first-in-man pilot clinical study. J Cosmet Laser Ther. 2018;20(6):369-9. [Google Scholar]
- Bhargava A, Singh V, Tiwari R. Revitalizing Hair Growth: A New Regimen Utilizing Growth Factor Concentrate for Hair Loss Treatment. Cureus. 2024;16(6). [Google Scholar] [Crossref]
- Shome D, Kapoor R, Doshi K, VK, Ram M. QR678 & QR678 Neo Hair Growth Formulations: A Cellular Toxicity & Animal Efficacy Study. Plast Reconstr Surg Glob Open. 2020;8(8). [Google Scholar] [Crossref]
- Kapoor R, Shome D, Vadera S, Ram M. QR678 & QR678 Neo vs PRP: A randomized, comparative, prospective study. J Cosmet Dermatol. 2020;19(11):2877-85. [Google Scholar]
- Kapoor R, Shome D, Surana M, Vadera S, RS. Efficacy of QR678 Neo® for the treatment of hair loss in telogen effluvium. J Cosmet Dermatol. 2022;21(1):16-23. [Google Scholar]
- Yano K, Brown L, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107(4):409-17. [Google Scholar]
- Li J, Zhang S, Zhao W, Sorbellini E. Comparative efficacy of hair growth formulations: PRP versus biomimetic peptides. J Dermatolog Treat. 2024;14(2):1671-82. [Google Scholar]
- Philpott M, Sanders D, Kealey T. Effects of insulin and insulin-like growth factors on cultured human hair follicles. J Invest Dermatol. 1994;102(6):857-61. [Google Scholar]
- Wang X, Liu Y, He J, Wang J, Chen X, Yang R. Regulation of signaling pathways in hair follicle stem cells. Mol Cell Biol. 2022;10. [Google Scholar] [Crossref]
- Shome D, Kapoor R. QR678® as an innovative solution for post-chemotherapy alopecia. J Cancer Surviv. 2022;16(3):123-34. [Google Scholar]
- Shapiro J, Simpson N. Hair regrowth treatments: Current options and future developments. Dermatol Clin. 2013;31(2):311-8. [Google Scholar]
- Kanchanapoomi S, Jirakranthayom T. Efficacy of biomimetic peptides in hair loss: A systematic review. Aesthetic Plast Surg. 2021;45(5):789-98. [Google Scholar]
- Ishida Y, Takahashi K, Ohki H. Advances in biomimetic peptides for clinical use. Biochem Soc Trans. 2017;45(1):203-12. [Google Scholar]
- Gupta M, Tiwari S. Comparative cost analysis of advanced alopecia therapies. J Dermatol Treat. 2023;34(1):45-53. [Google Scholar]
- Rao A, Mehta R. Innovative hair restoration techniques: A comprehensive review. Int J Trichology. 2024;16(1):13-22. [Google Scholar]
How to Cite This Article
Vancouver
Nazar J, Singhai S. Comparison between QR678 neo and platelet rich plasma for hair growth -A retrospective study [Internet]. J Dent Panacea. 2024 [cited 2025 Oct 06];6(4):193-196. Available from: https://doi.org/10.18231/j.jdp.2024.039
APA
Nazar, J., Singhai, S. (2024). Comparison between QR678 neo and platelet rich plasma for hair growth -A retrospective study. J Dent Panacea, 6(4), 193-196. https://doi.org/10.18231/j.jdp.2024.039
MLA
Nazar, Jincy, Singhai, Surabhi. "Comparison between QR678 neo and platelet rich plasma for hair growth -A retrospective study." J Dent Panacea, vol. 6, no. 4, 2024, pp. 193-196. https://doi.org/10.18231/j.jdp.2024.039
Chicago
Nazar, J., Singhai, S.. "Comparison between QR678 neo and platelet rich plasma for hair growth -A retrospective study." J Dent Panacea 6, no. 4 (2024): 193-196. https://doi.org/10.18231/j.jdp.2024.039
